PTI
TOKYO: In a first, researchers have created a comprehensive map of how the malaria parasite traffics between human host cells, an advance that sheds more light on the mechanisms behind parasitic infections of the blood.
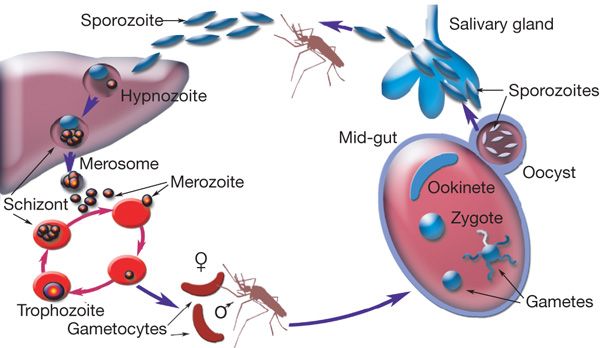
While malaria affects nearly 200 million people worldwide annually, the researchers, including those from Tohoku University in Japan, said the mechanism by which the disease-causing parasite effectively infiltrates human red blood cells was not thoroughly understood.
The study, published in the journal iScience, assessed how plasmodium falciparum — the parasite which causes the most severe form of malaria — infects red blood cells.
According to the researchers, P. falciparum triggers the production of several proteins within the host cell, transforming the cell’s physical form.
This transformation makes the cells stick in place, out of the body’s immune response, and also helps the parasite travel to the surface of the cell and infect others, they said.
These proteins, the study noted, help the parasite proliferate within the host, leading to the propagation of P. falciparum.
“Our study sheds light on the highly complicated interplay between parasite and host proteins in the host cytoplasm. The work provides a reliable dataset of the interactions connecting dozens of proteins the parasite exports to continue infecting the host cells,” said Kentaro Kato, study co-author from Tohoku University.
In the current study, the researchers looked at one of these proteins — skeleton-binding protein 1 (SBP1) — which is known to be highly important for malaria to propagate.
They imaged the way the parasite’s proteins interacted with SBP1 throughout the proliferation process, and identified several proteins connected to transforming the host cell.
“In this study, we developed an alternative approach to identify exported proteins involved in the trafficking complex and in the parasite protein exports. The SBP1 interactions established in our study represent a powerful and invaluable platform to identify exported proteins related to severe malaria caused by Plasmodium falciparum,” Kato said.
Based on the findings, the researchers developed a comprehensive map of SBP1’s interactions with other proteins, shedding light on the complex interplay between host and parasite proteins.
According to the scientists, the findings may pave the way for discussion on the molecular mechanism of the infections that affect human red blood cells.
The study, published in the journal iScience, assessed how plasmodium falciparum — the parasite which causes the most severe form of malaria — infects red blood cells.
According to the researchers, P. falciparum triggers the production of several proteins within the host cell, transforming the cell’s physical form.
This transformation makes the cells stick in place, out of the body’s immune response, and also helps the parasite travel to the surface of the cell and infect others, they said.
These proteins, the study noted, help the parasite proliferate within the host, leading to the propagation of P. falciparum.
“Our study sheds light on the highly complicated interplay between parasite and host proteins in the host cytoplasm. The work provides a reliable dataset of the interactions connecting dozens of proteins the parasite exports to continue infecting the host cells,” said Kentaro Kato, study co-author from Tohoku University.
In the current study, the researchers looked at one of these proteins — skeleton-binding protein 1 (SBP1) — which is known to be highly important for malaria to propagate.They imaged the way the parasite’s proteins interacted with SBP1 throughout the proliferation process, and identified several proteins connected to transforming the host cell.
“In this study, we developed an alternative approach to identify exported proteins involved in the trafficking complex and in the parasite protein exports. The SBP1 interactions established in our study represent a powerful and invaluable platform to identify exported proteins related to severe malaria caused by Plasmodium falciparum,” Kato said.
Based on the findings, the researchers developed a comprehensive map of SBP1’s interactions with other proteins, shedding light on the complex interplay between host and parasite proteins.According to the scientists, the findings may pave the way for discussion on the molecular mechanism of the infections that affect human red blood cells.